To read a Spanish translation of this story, visit the El País website.
When Marcelo Gordo opens the picnic cooler, the stench is suffocating. Three dead pied tamarin monkeys, their cream-and-caramel-colored coats visible through plastic wrap, are curled up inside. Gordo, a biologist at the Federal University of Amazonas, Manaus, explains that a student accidentally unplugged the freezer where he’d stored the monkeys, which had been killed on the road and given to him by city officials. Despite the decay, they are worth investigating.
Inside the spartan necropsy room at a veterinary school here, veterinarian Alessandra Nava and two graduate students pull on goggles, N95 masks, and blue nitrile gloves and begin to cut bits of tissue and collect bodily fluids from the monkeys. They pack the samples into vials to be transported to the Fiocruz Amazônia Biobank, a pathogen research collection that Nava helps oversee at the Amazonian regional office of the Oswaldo Cruz Foundation, a branch of Brazil’s Ministry of Health more commonly known as Fiocruz. There, she and others will test the samples for parasitic worms, viruses, and other infectious agents.
Nava and her colleagues are on the front lines of the search for animal diseases that could spill over and infect humans—and perhaps cause the next pandemic. New diseases can come from anywhere: Severe acute respiratory syndrome and COVID-19 both originated in China, for instance. Another recent coronavirus disease, Middle East respiratory syndrome, was first found in Saudi Arabia. But many researchers suspect tropical rainforests, with their staggering biodiversity, are the most likely cradle of dangerous new pathogens.

As a nonprofit journalism organization, we depend on your support to fund journalism covering underreported issues around the world. Donate any amount today to become a Pulitzer Center Champion and receive exclusive benefits!
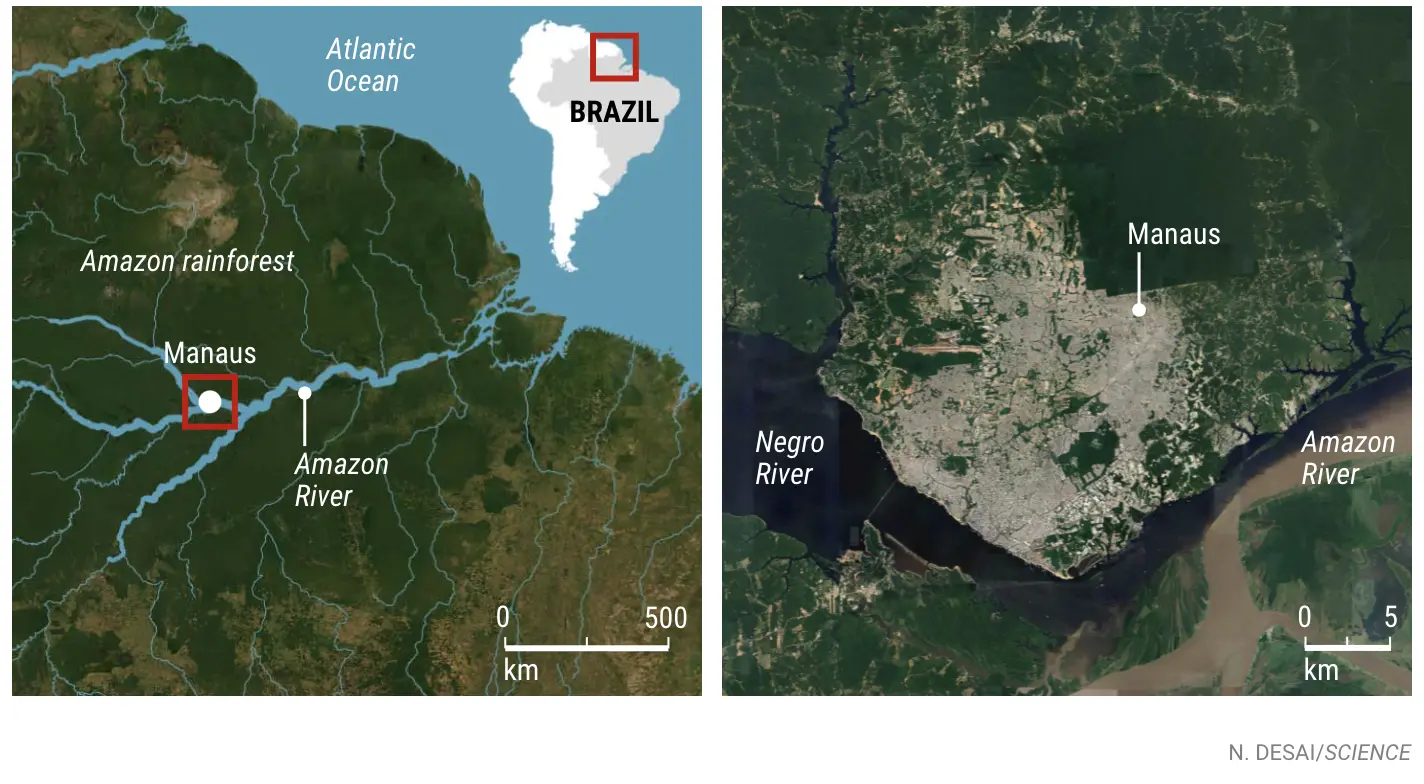
When human populations encroach on rainforests, the risk of spillover skyrockets. Manaus, Brazil, a city of 2.2 million people in the Amazon rainforest, is just such a place. The jungle that stretches for hundreds of kilometers in every direction has long threatened inhabitants with infections circulating in wildlife. Some 12% of the world’s 1400 bat species—known to host a bewildering range of viruses—flit through the Amazon forest. Its monkeys and rodents carry plenty of potential threats as well.

Urban growth, highway expansion, hydroelectric dam construction, mining for gold, and deforestation for cattle ranches and small farms erode the jungle and bring humans and wildlife into ever closer contact. In Brazil, the pro-business policies of President Jair Bolsonaro have only boosted that risk. By monitoring local animal populations and human patients, researchers at Fiocruz hope to head off zoonoses—diseases that leap from animals to humans—before they spiral out of control. Their work highlights the importance of curbing human activities that boost the risk of spillover. It could also guide surveillance for new and rare diseases in hospitals, which would enable health workers to respond fast if a rainforest pathogen became a wider threat.
Ironically, Fiocruz’s work has been stymied by one such disease. Manaus has experienced two brutal waves of COVID-19, a disease thought to have originated in bats. The city’s cumulative death toll, roughly 9000, is among the world’s highest per capita. Nava’s team has not captured animals at field sites in a year, partly out of concern that the researchers themselves might infect wild animals with the coronavirus. And the labs at Fiocruz Amazônia that process her samples have been commandeered for coronavirus research.

For Felipe Naveca, the lab’s vice director of research and innovation, the upheaval has been personal as well as professional. COVID-19 killed his father and may have contributed to the death of his grandmother. In the lab, Naveca led one of the first genetic studies of the new P.1 coronavirus variant that has emerged from Manaus and appears to be especially dangerous because it is more transmissible and evades immunity. He is proud that his team has processed 18,000 COVID-19 tests for local health authorities. “Helping to save someone’s life was much more rewarding than publishing a scientific article,” he says. But like his colleagues, Naveca is anxious to get back to the lab’s core mission. “We must keep searching for those emergent threats.”
THE OFFICE of Fiocruz Amazônia occupies a former military hotel in downtown Manaus, nestled between a small church and a luxury condominium high-rise. Several rooms with softly humming freezers and refrigerators house the biobank: a collection of feces, blood, and other tissues and fluids from more than 100 rainforest animals. Forty species are represented; the majority are monkeys, bats, and rodents, the mammals thought most likely to transmit disease to people. Other collections in the building include insects that torment these animals and could serve as vectors for ferrying pathogens to humans.
The Fiocruz Amazônia Biobank was partly modeled on the $200 million PREDICT early warning program. Launched in 2009 by the U.S. Agency for International Development, PREDICT identified nearly 1000 previously unknown animal viruses with zoonotic potential before the Trump administration canceled it in 2020. Whereas PREDICT was global, Nava and her colleagues do the same kind of work at a regional level. They’re searching for animal reservoirs of known viral and parasitic diseases, including obscure viral fevers and filariasis, a parasitic worm infection that can cause the horribly disfiguring syndrome elephantiasis. They’re also using DNA sequencers to scour samples from animals for pathogens that have yet to emerge.
“What they are doing is brilliant and important,” says Andrew Dobson, a biologist at Princeton University who studies the ecology of wildlife diseases. “It shows that even in countries with limited resources and a very negative governmental attitude towards science, it is possible to set up a monitoring scheme for novel viruses.”
Veteran disease hunter Dennis Carroll, who founded and ran PREDICT, agrees. “Amazonia is one of the richest, most ecologically diverse regions of the world,” he adds. “So getting any insight into that region is really important.”
Urban jungle
A black-and-white photograph in a second-floor foyer of Fiocruz Amazônia depicts one inspiration for this work: Brazil’s legendary doctor and disease sleuth, Carlos Chagas. Attired for an expedition in a white suit and knee-high boots, Chagas stands in a canoe surrounded by his oarsmen. In 1909, Chagas discovered the cause of the disease that now bears his name. Using a simple microscope, he identified the culprit as a protozoan (now called Trypanosoma cruzi) and showed that it is transmitted by the bite of triatomine bugs, often called kissing bugs. Chagas disease, whose symptoms range from fever to heart failure decades later, still kills hundreds of thousands of people a year in Latin America.
Naveca is doing similar detective work with the more sophisticated tools of modern genetics. One pathogen that concerns him is the little-studied Oropouche virus, which is spread primarily by a species of midge, Culicoides paraensis. Oropouche, which causes fever, headache, and joint pain, has sparked at least 30 outbreaks and sickened more than 500,000 people since it was first identified in 1955. Its range has gradually expanded to include Panama, six South American countries, and Trinidad and Tobago, where it first appeared. The midge itself, however, lives as far away as the northern United States, where it and related insects are called no-see-ums, suggesting the virus could spread beyond South America. The southern house mosquito (Culex quinquefasciatus), a carrier of West Nile and Saint Louis encephalitis viruses, can also transmit Oropouche, though not very efficiently, and its range throughout the tropics raises the possibility of Oropouche outbreaks in Africa, southeast Asia, and Australia.





Naveca and his colleagues hope to find out which animal or animals are the primary natural reservoirs for this virus. There are plenty of candidates: Oropouche has been identified in sloths, marmosets, finches, and several other birds and mammals. The team recently reported using the polymerase chain reaction to identify the virus’ genetic material in urine and saliva—as opposed to blood—which could make the hunt for its animal reservoir easier and aid diagnosis in patients.
Naveca is also worried about another little-studied virus that is rapidly expanding in South America: the Mayaro virus, which causes flulike symptoms, making it hard to distinguish from more common tropical diseases such as chikungunya and dengue fever. As with Oropouche, he’s hoping to pinpoint the virus’ natural reservoirs and investigate whether cases of it are going undiagnosed.
Mayaro is a likely candidate for the next large-scale outbreak of an animal virus in Brazil or beyond, Naveca and other scientists warn. Its primary vector, the mosquito Haemagogus janthinomys, is a forest dweller restricted to Central America and northern South America, but laboratory experiments show the yellow fever mosquito (Aedes aegypti) and the Asian tiger mosquito (A. albopictus)—two species widely distributed in tropical and subtropical areas—can also transmit the disease. A. aegypti is especially well adapted to breeding in cities.

To Naveca, the Zika virus is a case study in the value of tracking obscure pathogens. First identified in Africa in 1947, where it spilled over from monkeys, it circulated largely unnoticed and with few casualties for decades. Then, it caused an outbreak in Oceania in 2013 and, 18 months later, a massive epidemic in Latin America. Researchers suddenly discovered a disturbing consequence of the disease—microcephaly and other birth defects in infants born to infected mothers. “Zika was a virus that nobody was paying attention to until 10 years ago,” Naveca says. “We can fight better the enemies we know better.”
Naveca now hopes to carry on Chagas’s disease-hunting tradition with a deal he’s negotiating to procure a 25-meter, flat-bottomed boat that has been outfitted to be a floating laboratory. Preserving perishable human and animal samples at remote field sites has been a critical obstacle, and the vessel would bring the lab to the biological materials, rather than the other way around. Naveca hopes to join its maiden research voyage, possibly later this year, to remote Amazon villages, where he and colleagues plan to trap bats, rodents, primates, and insects, and bring a trove of specimens back to Fiocruz Amazônia.

EVEN WITHIN MANAUS, there are lots of opportunities for fieldwork. When Science visited last year, Gordo had set up an improvised lab inside a classroom at Sumaúma State Park, a tiny patch of uncut rainforest in the middle of the city, wedged between a busy highway and an upscale mall. Using cages baited with ripe bananas, he and his assistants trapped nine pied tamarins and injected them with a sedative, then swabbed their oral and anal cavities, clipped locks of hair, and drew blood. Then they set the animals free.
It’s peculiar and sometimes dangerous work. Monkeys have bitten and sneezed on Gordo, and on this trip a syringe broke as he squeezed the plunger, spraying monkey blood on his face shield. He says his wife complains when he stashes monkey carcasses in their home fridge.
Manaus’s Yoda-faced pied tamarins live all over the city. Like North American squirrels and raccoons, they don’t respect property lines and make urban gardens their pantries and playgrounds. There’s no evidence so far that Manaus’s urban monkeys are a human health threat, and Gordo, worried about “unreasonable killings or deforestation,” is reluctant to discuss that possibility. But he and others are investigating whether monkeys carry parasites, such as the nematodes that cause filariasis, or viruses such as Zika and chikungunya.
For Gordo, an equal concern is spillback—infections passed from humans to wildlife. Zika, for example, appears to have traveled from humans back to wild monkeys during Brazil’s epidemic. Fears that the virus might harm wildlife rose when researchers showed that a pregnant monkey native to Brazil had a spontaneous abortion after it was exposed to Zika. The fetus had birth defects similar to those seen in humans.
So far, Gordo has not found the virus in Manaus’s monkeys, but they may be at risk: A study he co-authored last year found mosquitoes from two species thought to carry Zika, Haemagogus janthinomys and Sabethes chloropterus, in both monkey and human habitats in a forest reserve on the edge of the city. The pied tamarins are already critically endangered, found nowhere else but in and around Manaus. Their population is expected to decline by 80% within the next 16 years. A virus outbreak could push them over the edge.

Humans are at risk from spillback as well. In Europe and the United States, scientists worry about COVID-19 outbreaks on mink farms, for example, because such events give the virus more opportunities to evolve and jump back into people. Likewise, primate populations infected with Zika could reignite human outbreaks. This happened with yellow fever: Brought to South America centuries ago with the slave trade, the virus has been impossible to eliminate from Brazil because it established itself in wild monkey populations, which occasionally pass it back to people.
After trapping monkeys for a day in the Sumaúma park, Gordo went home and bottle-fed an infant pale-throated sloth only slightly larger than his cupped hands. A friend had found it untended on the ground in a forest fragment not far from his university office. Despite everything he’s learned about zoonotic diseases, Gordo said he was “not too worried.” The sloth pup looked healthy. But several weeks later it got sick and died, possibly from pneumonia.
NAVA BELIEVES the Fiocruz center’s work is only becoming more urgent with changing land use patterns in the Amazon. Deforestation has soared since Bolsonaro came to power in 2019—transforming habitat in ways that could make viral hosts and vectors more dangerous and increasing the likelihood of spillover.
In 2016, she and colleagues reported that 9% of bats in small clearings around settlements in Brazil’s coastal Atlantic Forest had active infections of one or more of 16 viruses, including coronaviruses and hantavirus. In less-disturbed forests nearby, fewer than half as many bats were infected, and with only six different viruses. The findings fit a widely debated hypothesis known as the dilution effect, which holds that in forests with greater biodiversity, mosquitoes and other vectors have more targets and end up biting animals not capable of incubating a given virus, thereby slowing its spread. Reducing biodiversity by clearing land can do the opposite, and it also pushes humans into closer proximity to wildlife. Bats are a particular concern, Nava says, because they often roost in buildings.
It all underscores the need to stop destroying rainforest, she says—although she acknowledges that Brazil’s policies are unlikely to change under Bolsonaro, who has nearly 2 years left in his term. In the meantime, Nava says, disease fighters must keep monitoring the jungle for dangerous diseases. “We have no power to reduce deforestation,” she says. But, she adds, “We have the power to search for new viruses.”